- Serial Dilution Practice Problems
- Series Dilution Table
- Serial Dilution Experiment
- Serial Dilution Calculator
Table of Contents. How to Do a Viable Plate Count; 1: Why we need to dilute; 2: Setting the stage: Diluting Cold Press Coffee; 3: The dilution factor; 4: Overall dilution; 5: Design a dilution! 6: How to scale back up; 7: Count a plate! 8: Why plate more than one dilution? 9: Picking the plate count; Section 2. Practice Problems; 10. Microbial monitoring with serial dilution culture media. LifeCheck Media (also referred to as culture media, bug bottles or nutrient broths) is a microbial testing technology based upon growing bacteria. These tests work by adding a volume of liquid sample to a vial with a food source that allows specific microbes to grow (ie.
1 mL coffee + 4 mL water =. 1 mL coffee + 9mL water =. 1 mL + 99mL water =. As you've probably guessed, this works exactly the same whether you're talking about caffeine or meningicocci. Here's what a dilution factor of 0.01 looks like on the lab bench: Notice that it really doesn't matter how much of the original stock you started with, as.
Calculate the volumes required to prepare a serial dilution for an assay. Generates a step-by-step protocol for planning serial dilutions. Calculates serial dilution using initial concentration and dilution factor or a concentration range. Please select the document or documents you would like from the list below. We appreciate your support of our products and hope that you find this service helpful to your clinical practice.
Introduction
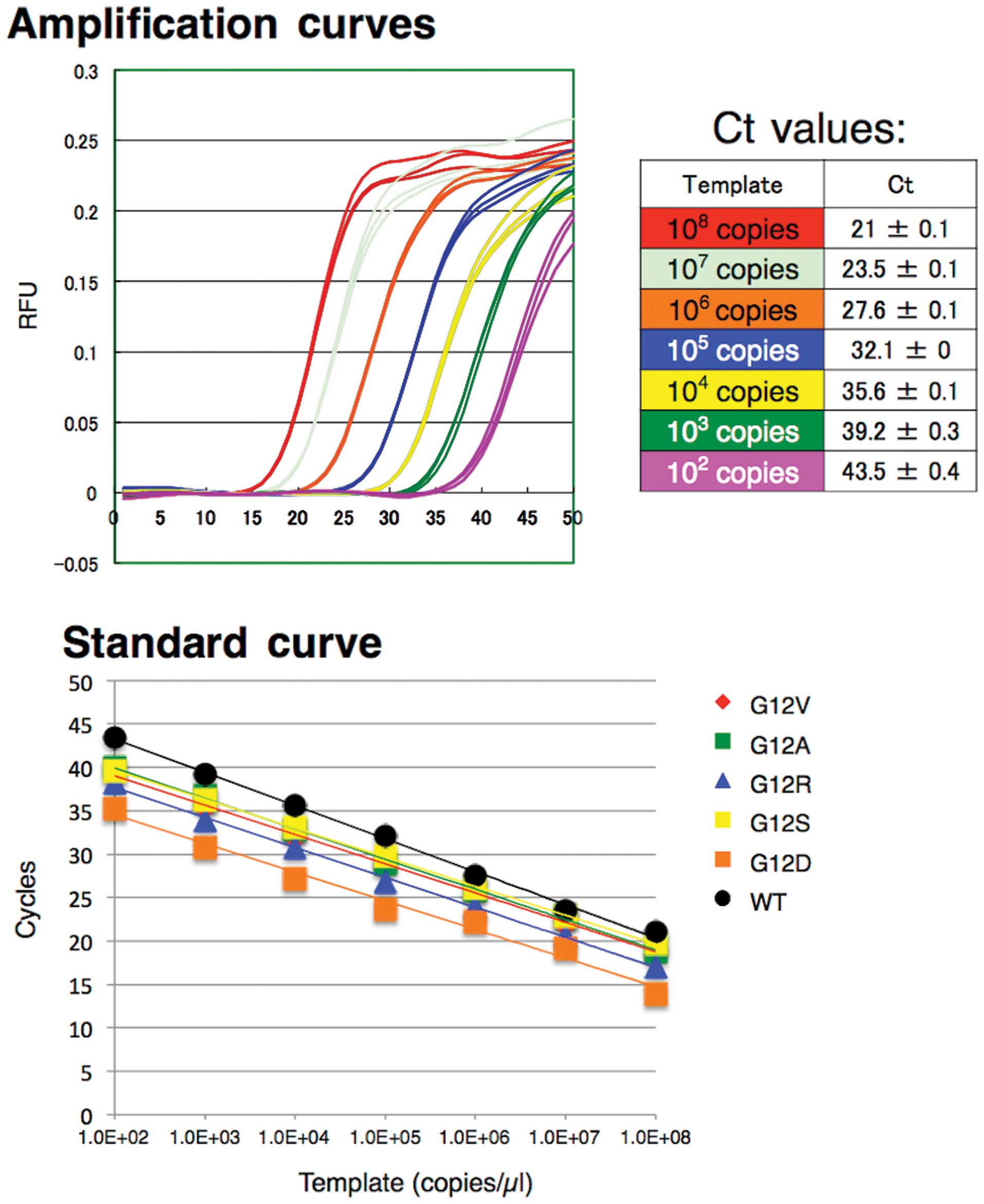
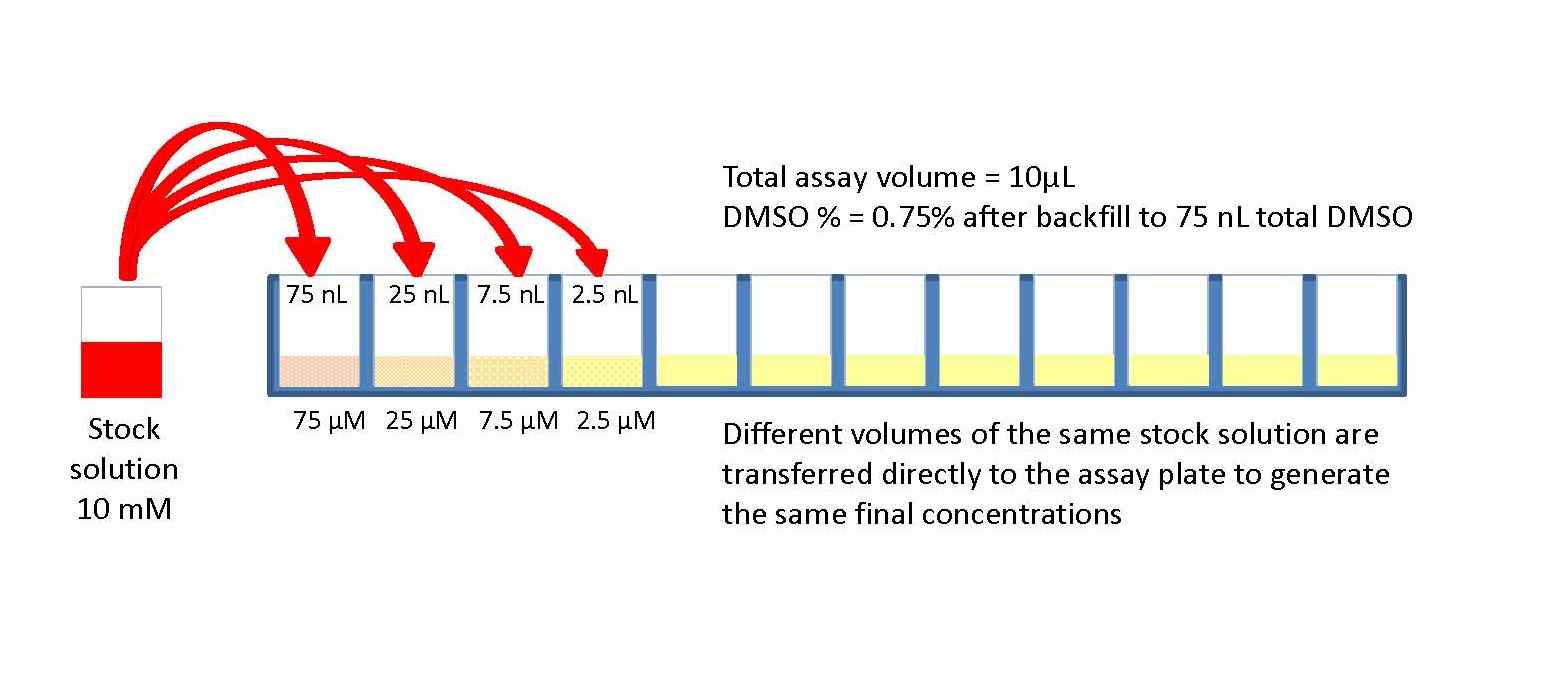
A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The concentration factor is the initial volume divided by the final solution volume; the dilution factor would be the inverse of the concentration factor. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; this is 1/10th (0.1) of the concentration of the original solution and has a dilution factor of 10. These serial dilutions are often used to determine the approximate concentration of an enzyme (or molecule) to be quantified in an assay. Serial dilutions allow for small aliquots to be diluted instead of wasting large quantities of materials, are cost-effective, and are easy to prepare.
Equation 1.
[concentration factor= frac{volume_{initial}}{volume_{final}}nonumber]
[dilution factor= frac{1}{concentration factor}nonumber]
Diagram of 1:2 Serial Dilutions
In your notebook, draw a diagram showing the serial dilutions for the 6 KMnO4 solutions you are preparing. In the diagram, indicate the volume being withdrawn from the concentrated solution, the volume of water added, the concentration of the new solution, and the total volume.
Learning Objective
- Calculate the concentration of a diluted solution.
Key Points
- Most commonly, a solution’s concentration is expressed in terms of mass percent, mole fraction, molarity, molality, and normality. When calculating dilution factors, it is important that the units of volume and concentration remain consistent.
- Dilution calculations can be performed using the formula M1V1 = M2V2.
- A serial dilution is a series of stepwise dilutions, where the dilution factor is held constant at each step.
Terms
- dilutiona solution that has had additional solvent, such as water, added to make it less concentrated
- serial dilutionstepwise dilution of a substance in solution
Dilution refers to the process of adding additional solvent to a solution to decrease its concentration. This process keeps the amount of solute constant, but increases the total amount of solution, thereby decreasing its final concentration. Dilution can also be achieved by mixing a solution of higher concentration with an identical solution of lesser concentration. Diluting solutions is a necessary process in the laboratory, as stock solutions are often purchased and stored in very concentrated forms. For the solutions to be usable in the lab (for a titration, for instance), they must be accurately diluted to a known, lesser concentration.
The volume of solvent needed to prepare the desired concentration of a new, diluted solution can be calculated mathematically. The relationship is as follows:
[latex]M_1V_1=M_2V_2[/latex]
Serial Dilution Practice Problems
M1 denotes the concentration of the original solution, and V1 denotes the volume of the original solution; M2 represents the concentration of the diluted solution, and V2 represents the final volume of the diluted solution. When calculating dilution factors, it is important that the units for both volume and concentration are the same for both sides of the equation.
Example
- 175 mL of a 1.6 M aqueous solution of LiCl is diluted with water to a final volume of 1.0 L. What is the final concentration of the diluted solution?
- [latex]M_1V_1=M_2V_2[/latex]
- (1.6 M)(175 mL) = M2(1000 mL)
- M2 = 0.28 M
Serial Dilutions
Serial dilutions involve diluting a stock or standard solution multiple times in a row. Typically, the dilution factor remains constant for each dilution, resulting in an exponential decrease in concentration. For example, a ten-fold serial dilution could result in the following concentrations: 1 M, 0.1 M, 0.01 M, 0.001 M, and so on. As is evidenced in this example, the concentration is reduced by a factor of ten in each step. Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale. Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.
Show SourcesSeries Dilution Table
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/serial%20dilution
Wikipedia
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/dilution
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Serial_dilution
Wikipedia
CC BY-SA 3.0.
Serial Dilution Experiment
Serial Dilution Calculator
http://cnx.org/content/m17123/latest/
OpenStax CNX
CC BY 3.0.
http://commons.wikimedia.org/wiki/File:Dilution-concentration_simple_example.jpg
Wikimedia
CC BY-SA.



